The ADVENTa Clinical Trial
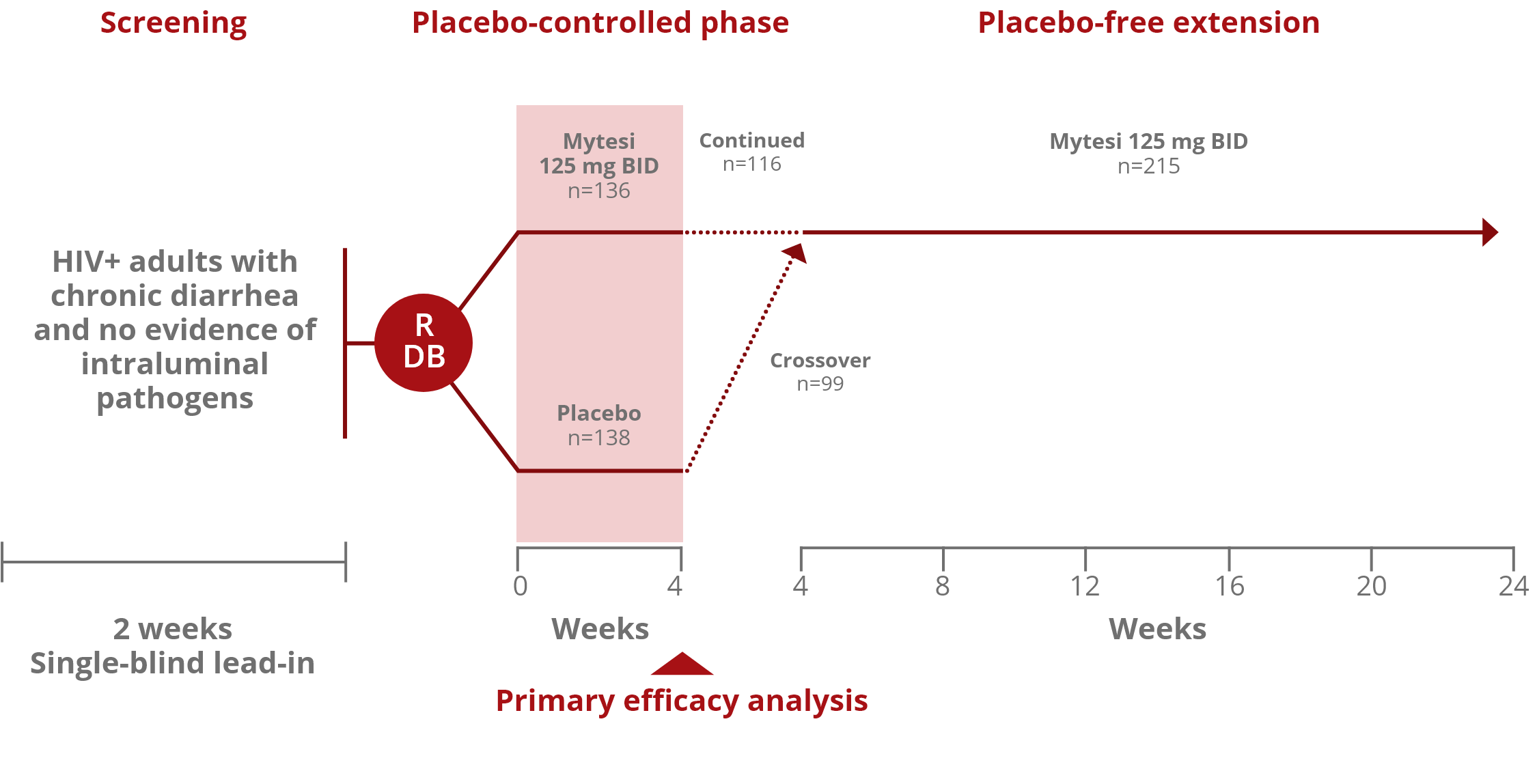
Mytesi (crofelemer) 125 mg taken twice daily was approved based on the ADVENT clinical trial, a double-blind, placebo-controlled study with a 2-stage adaptive design. The trial included 374 people living with HIV (PLWH) who had noninfectious diarrhea for 1 month or more and were on antiretroviral therapy.1,2,b

Study Design
Mytesi demonstrated a significant decrease in
diarrhea in the ADVENT clinical trial2,3
Reduction in number of watery stools per week by
crofelemer treatment week3
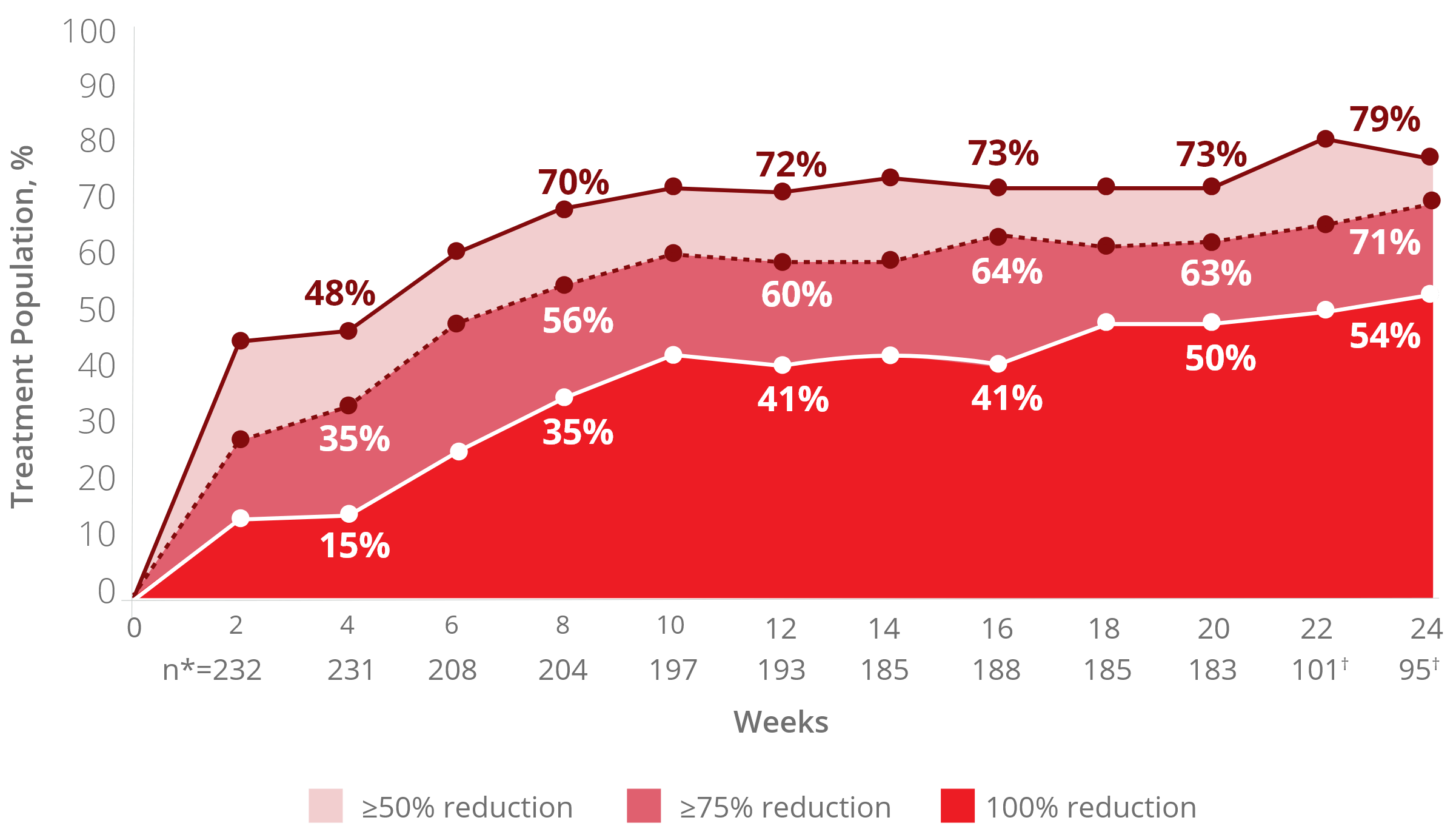
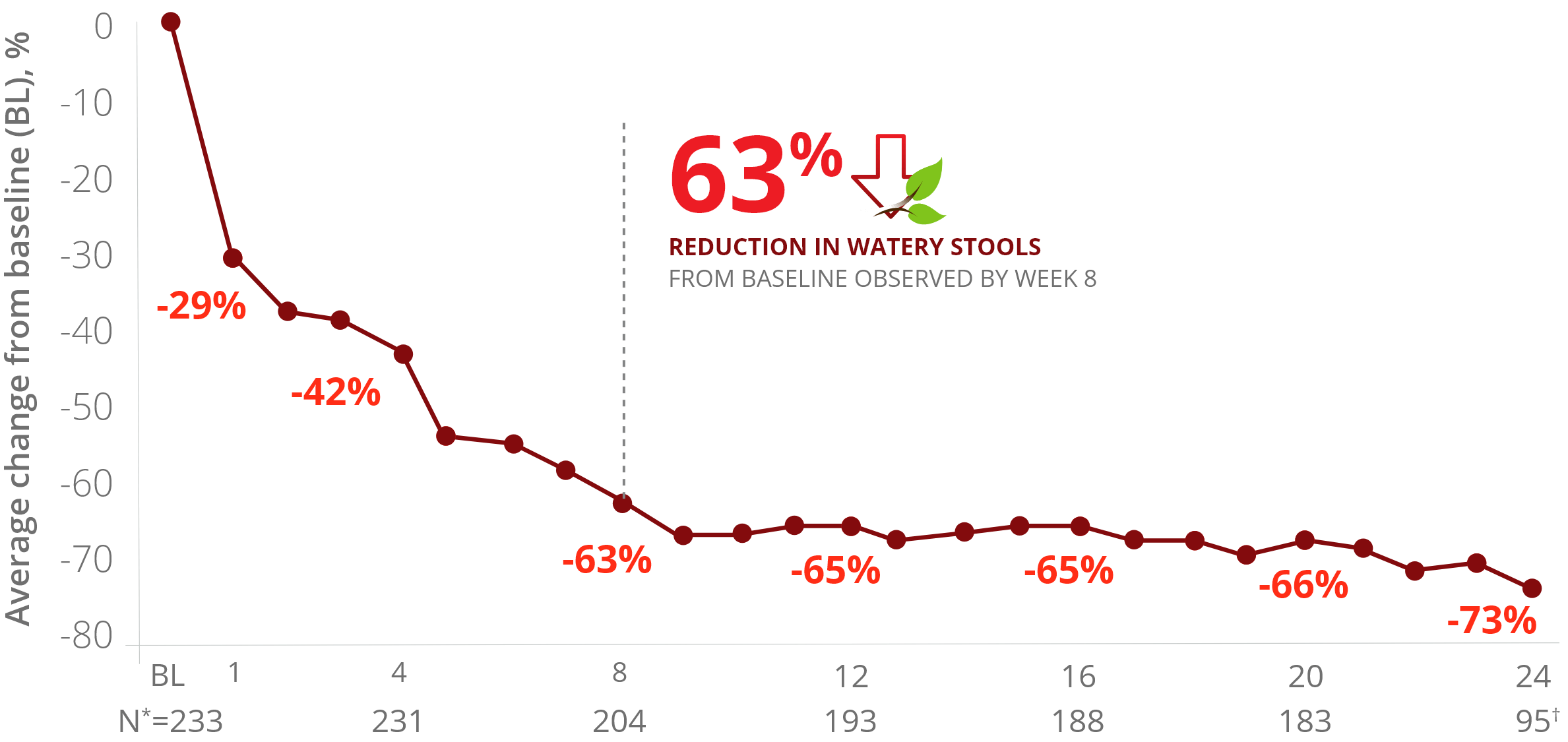
*Number of patients each week with evaluable diary data.
†Weeks 21-24 include data only from patients initially randomized to crofelemer.
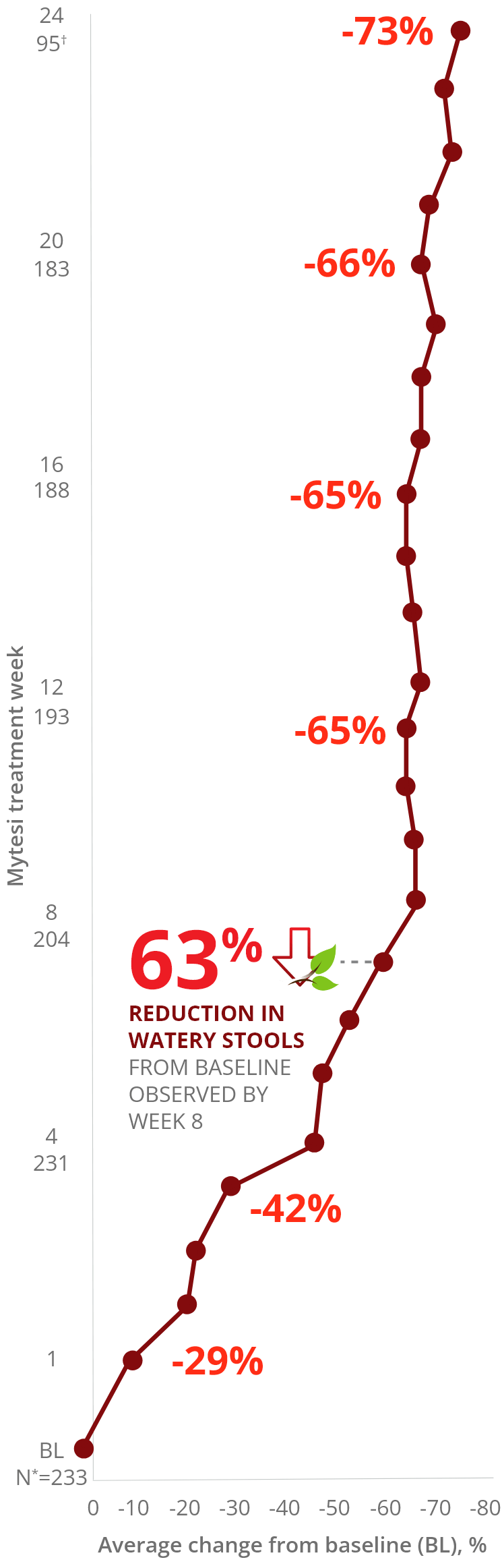
Reduction in Watery Stools Over Time with Mytesi3
Mytesi treatment week
ITT population: 125 mg BID (n=136), 250 mg BID (n=54), 500 mg BID (n=46), Placebo BID (n=138).
*Number of patients each week with evaluable diary data.
†Weeks 21-24 include data only from patients initially randomized to Mytesi (crofelemer).
ITT population: 125 mg BID (n=136), 250 mg BID (n=54), 500 mg BID (n=46), Placebo BID (n=138).
*Number of patients each week with evaluable diary data.
†Weeks 21-24 include data only from patients initially randomized to Mytesi (crofelemer).
The reduction in watery stools decreased gradually
and improved the longer a patient stayed on therapy.3
The reduction in watery stools decreased gradually
and improved the longer a patient stayed on therapy.3
ADVENT Clinical Trial
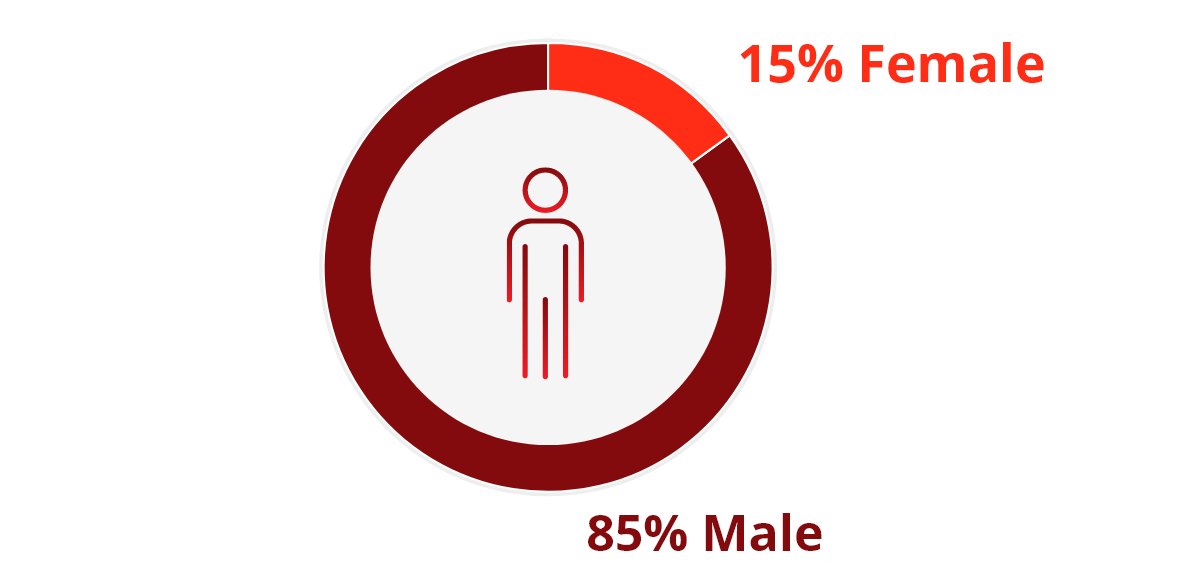
Population Characteristics1,2
The ADVENT clinical trial included 374 HIV+ patients with noninfectious diarrhea for ≥1 month who were stable on ART (for ≥4 weeks prior to screening) with CD4+ T cell counts ≥100 cells/μL.c




The trial participants were:

aAntidiarrheal Therapy in HIV Disease – Emerging Treatment Concepts.
bThe ADVENT clinical trial included HIV+ adults with chronic diarrhea and no evidence of intraluminal pathogens.
cDiarrhea was defined as either persistently loose stools despite regular use of antidiarrheal medication, or one or more watery bowel movements per day without regular antidiarrheal medicine use.
References:
1. Mytesi [package insert]. San Francisco, CA: Napo Pharmaceuticals. 2018. 2. MacArthur R, Harkins TN, Brown SJ, et al. Efficacy and safety of Crofelemer for non-infectious diarrhea in HIV-seropositive individuals (ADVENT Trial): a randomized, double-blind, placebo-controlled, two-stage study. HIV Clin Trials. 2013;14(6):261-273. 3. MacArthur RD, Clay P, Blick G, Waltzman R, Bell M. Long-term Crofelemer provides clinically relevant reductions in HIV-related diarrhea. 9th IAS Conference on HIV Science (IAS 2017), July 23-26, 2017, Paris, France. Abstract WEPEB0537.



