Mytesi is well tolerated, with a low incidence of adverse events
The ADVENT clinical trial reported a low rate of adverse events, compared to placebo.



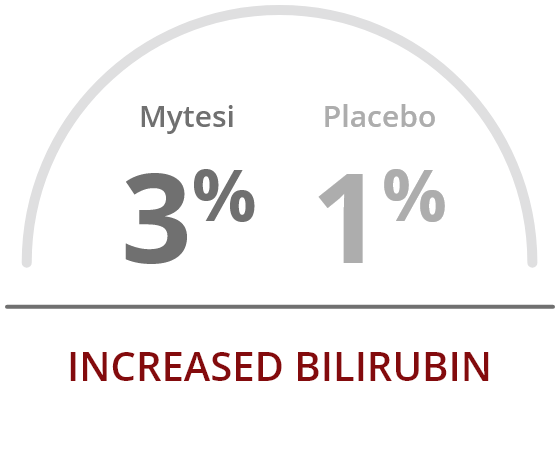
The most common adverse reactions occurring at a rate greater than placebo were2:
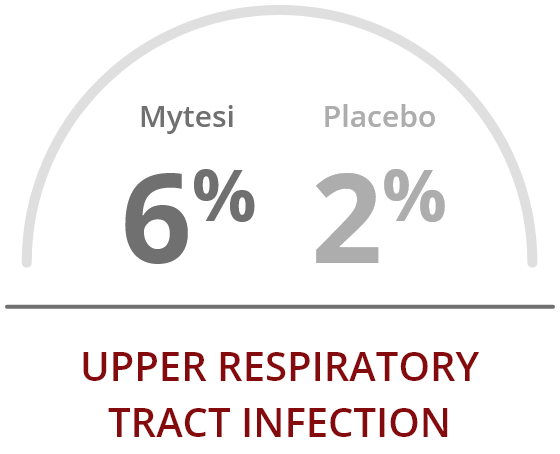
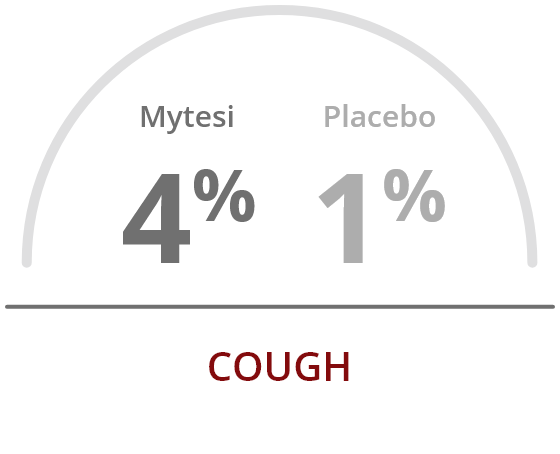
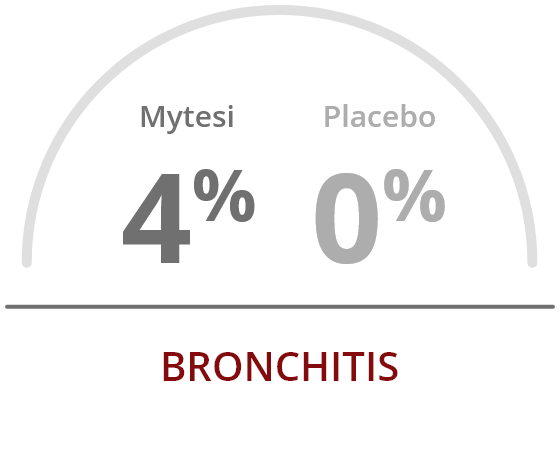
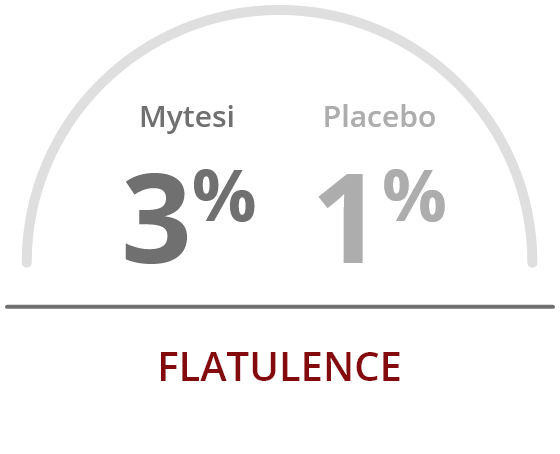
References:
1. Mytesi [package insert]. San Francisco, CA: Napo Pharmaceuticals. 2018. 2. MacArthur R, Harkins TN, Brown SJ, et al. Efficacy and safety of Crofelemer for non-infectious diarrhea in HIV-seropositive individuals (ADVENT Trial): a randomized, double-blind, placebo-controlled, two-stage study. HIV Clin Trials. 2013;14(6):261-273.




